Visualizing phase diagrams#
This section was contributed by Haoyuan Li and Magali Billen.
For many models it is useful to see how the material parameters vary as a function of pressure and temperature. In this cookbook, a plot of material properties (e.g., density or viscosity) as a function of temperature (x axis) and pressure (y axis) is referred to as a phase diagram.
In this phase diagram cookbook, phase diagrams are generated from model runs and visualized with VisIt, but ParaView could also be used to view the same output. This is a simple way of using the visualization output to check the correct implementation of phase transitions in a material model. The idea lies in using a model with an initial temperature that increases linearly along the \(x\)-axis and a pressure that changes linearly along the \(y\)-axis in a box geometry, so that the two axes, \(x\) and \(y\), can be interpreted as axes for pressure and temperature in the graphical output. Here, we visualize a diagram of the phase transitions implemented in the visco plastic material model, as well as a lookup table in the Steinberger material model.
The input file#
You can find the input file to run this cookbook example in cookbooks/visualizing_phase_diagram/visualizing_phase_diagram.prm. For this first case, phase transitions are prescribed manually in terms of their depth, Clapeyron slope, and other key parameters.
The model domain is 800 km by 800 km box. Initial temperature increases from 273 K on the left side to 2273 K on the right side. Pressure, on the other hand, increases from the top of the box to the bottom with a constant gradient. This is assured by assigning a constant density with zero expansivity. It differs from a classic phase diagram only in the direction of pressure increase.
Two compositions of pyrolite and harzburgite are included in the model. With each run, only one composition is assigned to the whole domain in order to visualize the diagrams of these two separately.
The material model is then set up to mimic mantle phase transitions at 410, 520, and 660 km. Details of phase transitions are taken from Billen and Arredondo [2018]. A trick is needed to make this work: we assign the values of the density to the field of heat capacity in the input file. To do this, We use the phase inputs implemented in the visco plastic plug-in. This serves the goal of visualizing values of reference densities of phases with the real density assigned with a constant value in the model. Inputs for this material model are listed here:
subsection Material model
set Model name = visco plastic
subsection Visco Plastic
set Reference temperature = 273
set Maximum viscosity = 1e24
set Phase transition depths = background:410e3|520e3|560e3|670e3|670e3|670e3|670e3, spharz: 410e3|520e3|560e3|670e3|670e3|670e3|670e3
set Phase transition widths = background:5e3|5e3|5e3|5e3|5e3|5e3|5e3, spharz: 5e3|5e3|5e3|5e3|5e3|5e3|5e3
set Phase transition temperatures = background:1662.0|1662.0|1662.0|1662.0|1662.0|1662.0|1662.0, spharz: 1662.0|1662.0|1662.0|1662.0|1662.0|1662.0|1662.0
set Phase transition Clapeyron slopes = background:4e6|4.1e6|4e6|-2e6|4e6|-3.1e6|1.3e6, spharz: 4e6|4.1e6|4e6|-2e6|4e6|-3.1e6|1.3e6
set Densities = 3300.0
set Heat capacities = background: 3300.0|3394.4|3442.1|3453.2|3617.6|3691.5|3774.7|3929.1, spharz: 3235.0|3372.3|3441.7|3441.7|3680.8|3717.8|3759.4|3836.6
set Thermal expansivities = 0.0
end
end
Results#
Visualization of the model results yields a phase diagram of a pyrolitic mantle (Fig. 71). The field shown here has the reference densities of the pyrolite phases, though settings of phase transitions are over-simplified. One may notice that three transitions (i.e., one in the olivine system, two in the spinel system) are included for the 660 interfaces, and they need to be modified at a higher temperature. In spite of the complexities of mantle phases, the focus of this first example is to simply illustrate this approach of visualizing it. Beyond showing the diagram, We have also used the “lineout” feature in VisIt to export the data along two vertical lines at \(T = 1173\text{ K}\) and \(T = 1673\text{ K}\) (Fig. 72). The figure for \(T = 1173\text{ K}\) illustrates the buoyancy forces felt by a descending cold slab within the mantle transition zone.
Next, we shift to harzburgite by changing the values of the initial compositional field from 0 to 1. For this to occur, the following changes are needed:
# initial composition model
subsection Initial composition model
set List of model names = function
subsection Function
set Coordinate system = cartesian
set Function expression = 1.0 # change this to one
end
end
With this change, we can also visualize the phase diagram of harzburgite in Fig. 71.
Moreover, We tested the pyrolitic lookup table used in the Steinberger material model (Fig. 73). The same setup of the initial condition is applied as in the previous case. The densities, however, are not assigned to the heat capacity anymore. Thus the vertical axis would deviate from the axis of pressure a little bit. This second setup serves the goal of illustrating a more complex and thus more realistic model of phase transitions. Modification for the material model is listed below:
subsection Material model
set Model name = Steinberger
subsection Steinberger model
set Data directory = $ASPECT_SOURCE_DIR/data/material-model/steinberger/
set Lateral viscosity file name = temp-viscosity-prefactor.txt
set Material file names = pyr-ringwood88.txt
set Radial viscosity file name = radial-visc.txt
set Maximum lateral viscosity variation = 1e2
set Maximum viscosity = 1e23
set Minimum viscosity = 1e19
set Use lateral average temperature for viscosity = true
set Number lateral average bands = 10
set Bilinear interpolation = true
set Latent heat = false
end
end
Compared to the first example, pyrolitic phases are illustrated on a much finer scale. Meanwhile, we can still tell the phase transitions at 410, 520, and \(660\text{ km}\) depth, respectively, marked by linear boundaries analogous to a constant Clapeyron slope.
.
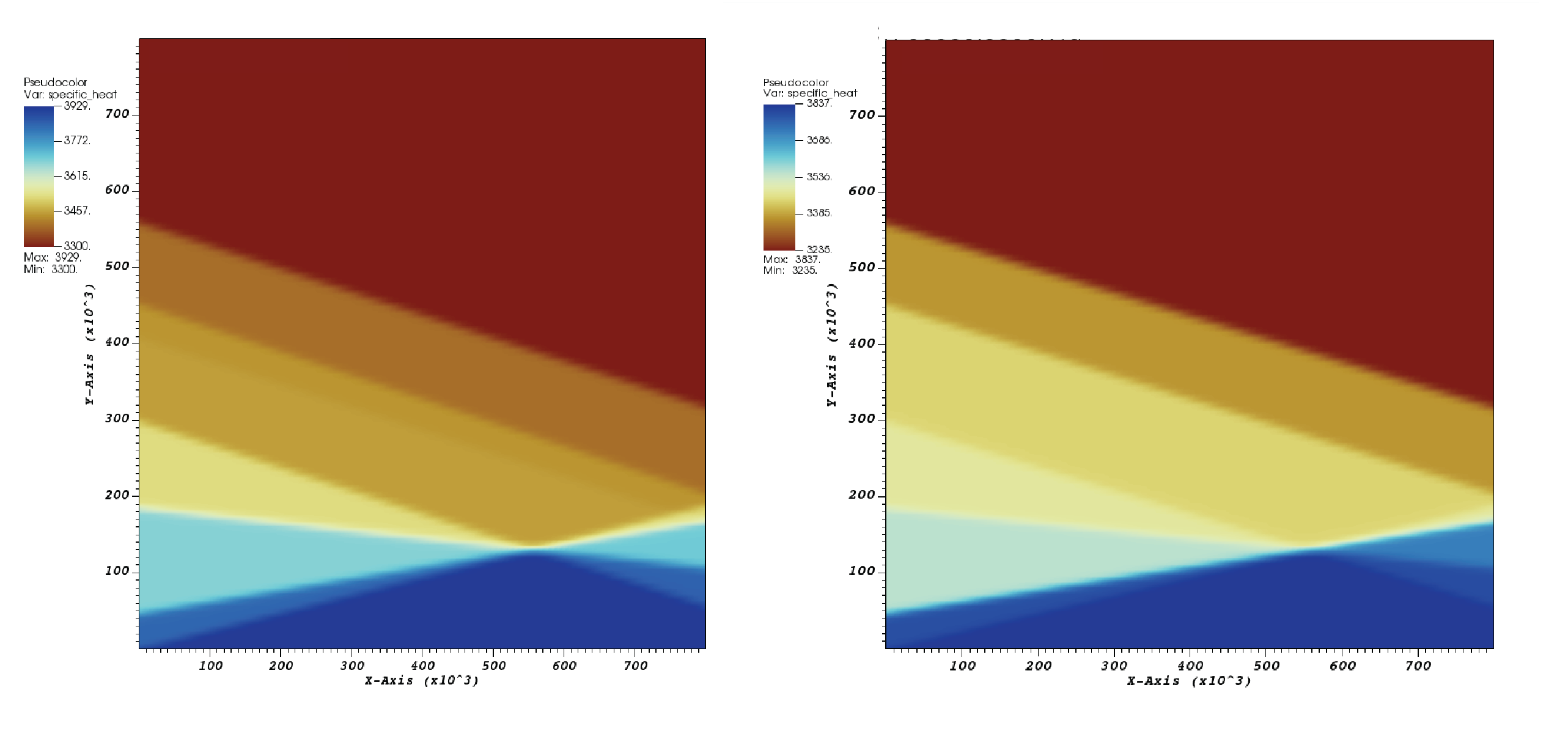
Fig. 71 Visualization of phase diagrams: The field of heat capacity showing values of reference densities for pyrolitic and harzburgitic phases.#
.
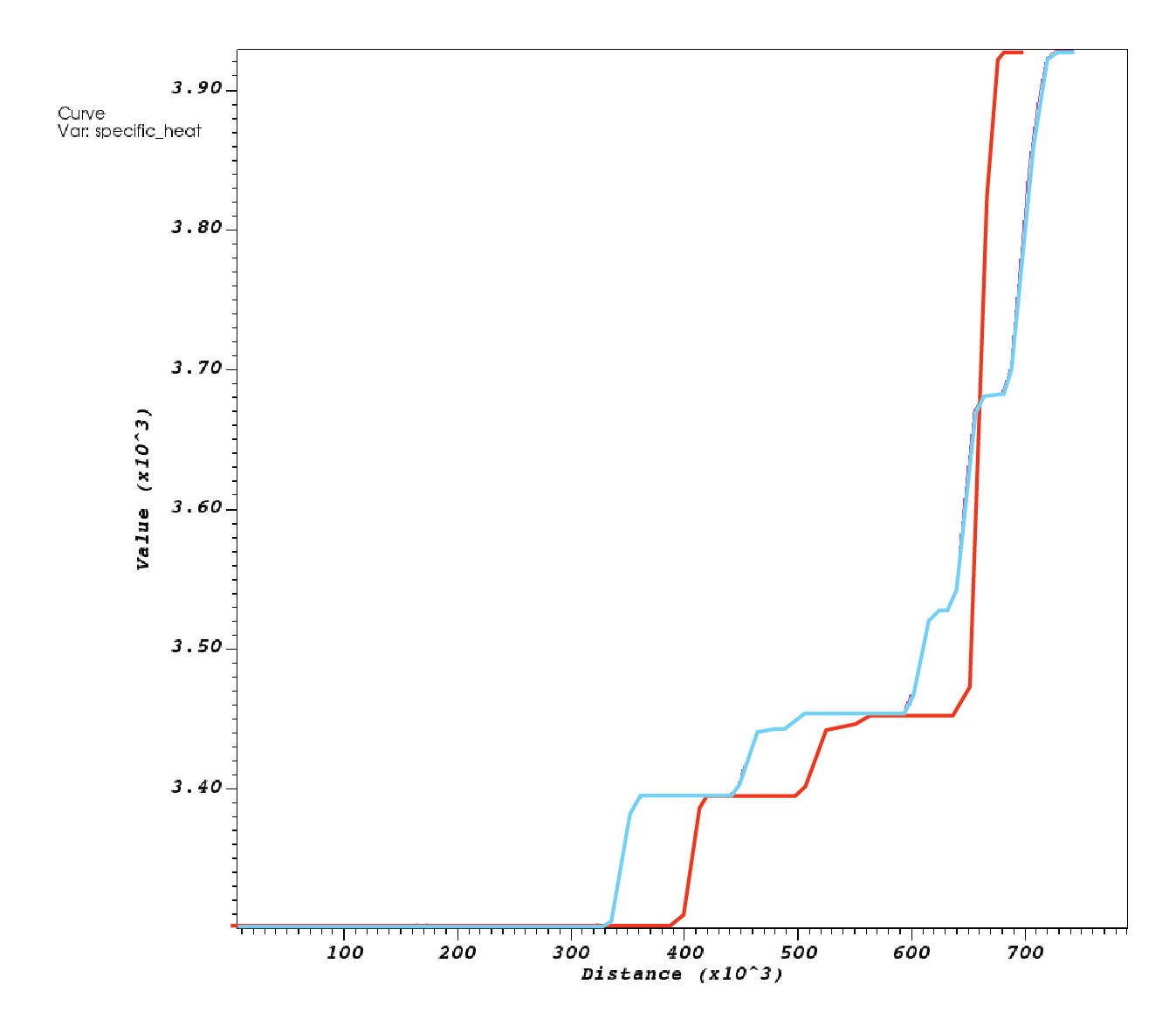
Fig. 72 Visualization of phase diagrams: Profiles of pyrolitic density at \(T=1173\text{ K}\) (red) and \(1673\text{ K}\) (blue).#
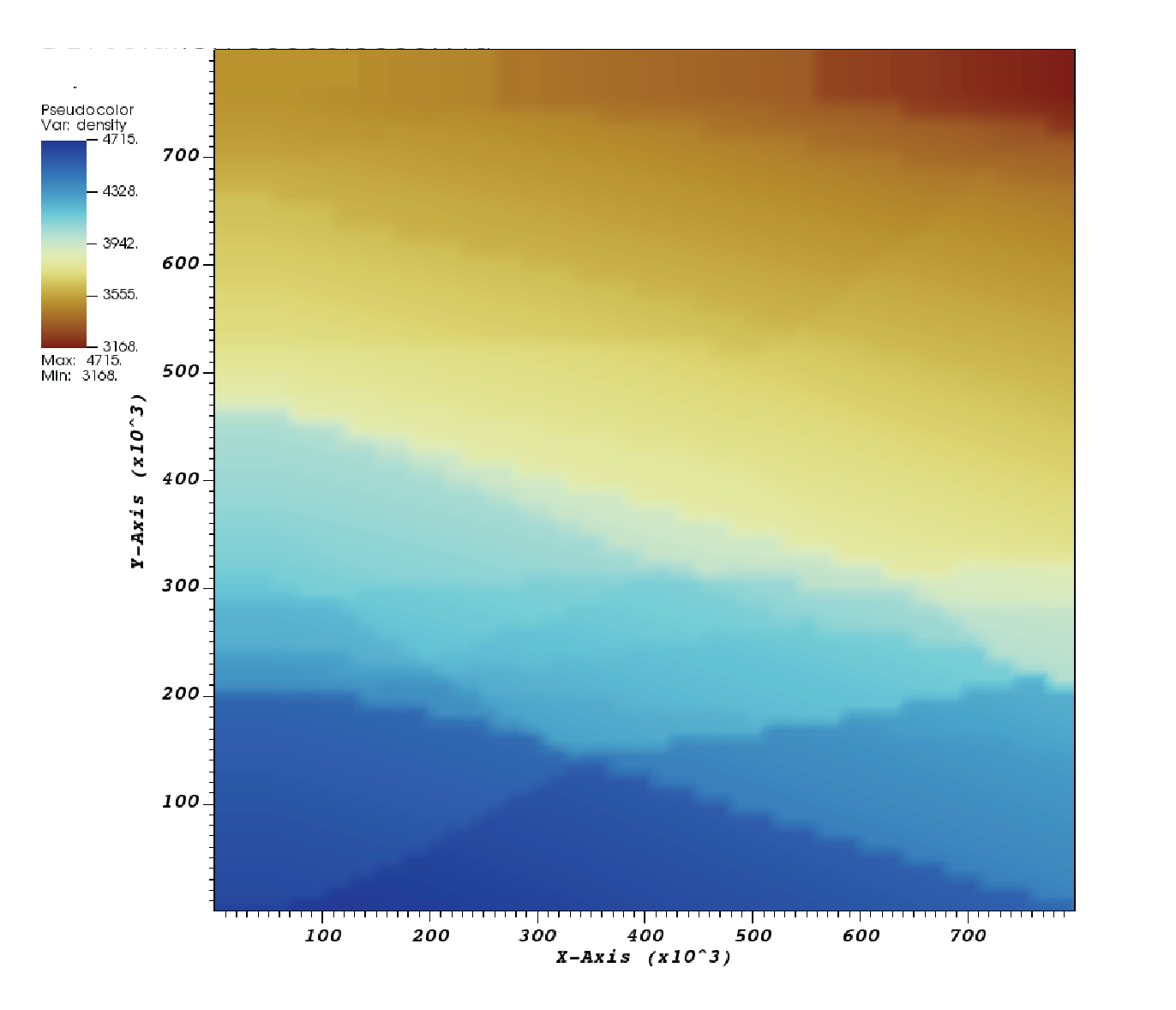
Fig. 73 Visualization of phase diagrams: Density from lookup table of pyrolite from Stixrude and Lithgow-Bertelloni [2011].#